TRANSFORMATIVE TREATMENTS
Focused on the Whole You.
TRANSFORMATIVE
TREATMENTS
Focused on the Whole You
Synergistic Modalities Unlock Your Best Self
Experience an integrative, holistic approach, tailored to meet the needs of
your brain, your body, and the way you interact with the world around you.

Brain Health
Infusions to calm and nourish your nervous system and programs to activate self-directed neuroplastic changes.

Body Health
Nutrient and vitamin infusions to help your body feel more resilient and resourced.

Weight Loss
Medically supported weight loss.
Prescription medications that work.
YOU'VE GOT THIS! We've got you.

Integration
Integration brings the parts together
to make a whole. Focused modalities facilitate transformative and lasting personal and habitual changes.
Sustainable Wellbeing
Combine positive daily behaviors with a mindset oriented toward growth, self-love, and community.
Sustainable well-being arises when we prioritize physical health through robust nutrition and mindful movement, build supportive communities that offer connection and purpose, and cultivate nurturing and resilient behavioral patterns like emotional regulation and healthy coping strategies. This holistic approach fosters achievable and sustainable well-being where you are the foundation and your interactions with the community around you become the support system to sustaining well-being.
Whole Body Programs
Nourish, Empower, Thrive:
Your personalized well-being journey starts here.
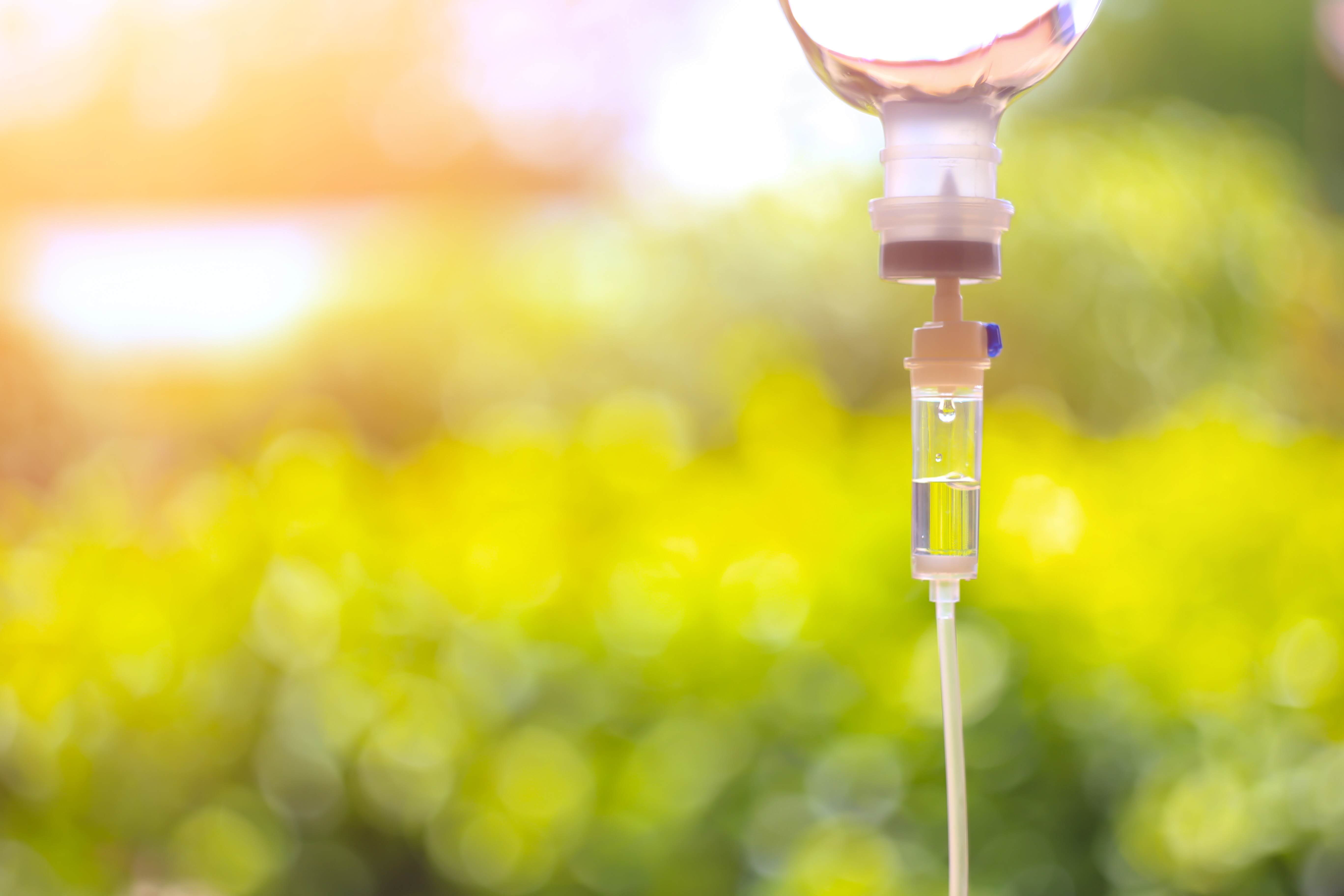
IV Infusions
Personalized care plans, adjusted to meet your needs at each session; Vitamins, Magnesium, Glutathione, NAD+, and others.

Weight Loss
Weight loss programs tailored for you, with medically supported options available.

Integrative Coaching
Ongoing support along your path.
Cultivate key life skills for long-lasting
growth and development.

Supported Sessions
Compassionate and experienced coaches and advocates maximize your comfort, preparation, and integration.

IV Nutrients

Functional Medicine
Support For Your Whole Journey
We're ready to support your whole health and wellbeing.
Come as you are, and experience transformative healing, repair, health, and sustained wellness for your Whole Body.
Synergistic Modalities Unlock Your Best Self
Experience an integrative, holistic approach, tailored to meet the needs of your brain, your body, and the way you interact with the world around you.

Brain Health
Infusions to calm and nourish your
nervous system and programs to activate
self-directed neuroplastic changes.

Body Health
Nutrient and vitamin infusions to help
your body feel more resilient and resourced.

Weight Loss
Medically supported weight loss.
Prescription medications that work.
YOU'VE GOT THIS! We've got you.

Integration
Integration brings the parts together to make a whole. Focused modalities facilitate transformative and lasting personal and habitual changes.
Sustainable Wellbeing
Combine positive daily behaviors with a mindset oriented toward growth, self-love, and community.
Sustainable well-being arises when we prioritize physical health through robust nutrition and mindful movement, build supportive communities that offer connection and purpose, and cultivate nurturing and resilient behavioral patterns like emotional regulation and healthy coping strategies. This holistic approach fosters achievable and sustainable well-being where you are the foundation and your interactions with the community around you become the support system to sustaining well-being.
Whole Body Programs
Nourish, Empower, Thrive:
Your personalized well-being journey starts here.

IV Infusions
Personalized care plans, adjusted to meet your needs at each session; Vitamins, Magnesium, Glutathione, NAD+, and others.

Weight Loss
Weight loss programs tailored for you, with medically supported options available.

Integrative Coaching
Ongoing support along your path.
Cultivate key life skills for long-lasting
growth and development.

Supported Sessions
Compassionate and experienced coaches and advocates maximize your comfort, preparation, and integration.

IV Nutrients


